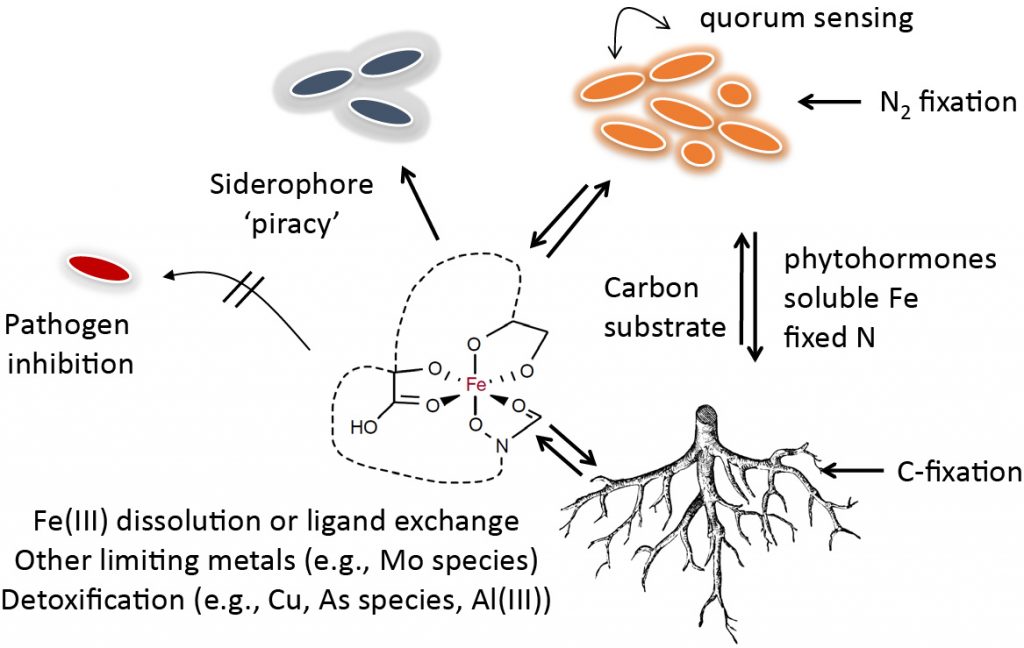
Microbiomes in the environment can be expected to be significantly controlled by the availability of nutrients and therefore by cooperation – or competition – to use these resources for biological growth. Iron is a critical micro-nutrient with extremely low availability at neutral or basic pH values. Acidic soils, on the other hand, can often carry a high concentration of toxic metals. Little is known about the importance of trace-metal limitation or toxicity in the field – specifically, there is nearly no insight into the distribution of secreted microbial and fungal iron shuttles (siderophores) and other metal sequestering compounds in the field: what are their structures and how are they used, what is their role in plant pathology, do they play a role in metal detoxification and uptake of other trace-metals? Since iron limitation in soil is a fundamental kinetic problem, what are the rates with which secreted molecules can solubilize and shuttle iron during growth?
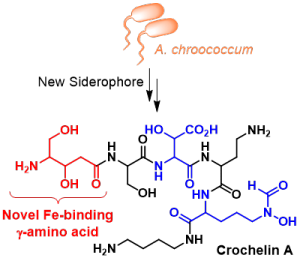
We developed chelomics – a new metabolomic approach for discovery and characterization of metal chelating compounds in complex samples. The application of chelomics has revealed unprecedented siderophore chemistries and large families of structurally related compounds originating from a single biosynthetic gene cluster. Specific chelators may be secreted to take up trace-metals other than iron, or to detoxify heavy metals. Extending these fundamental studies about soil trace-metal microbiology and chemistry, we are expanding our research for system-wide investigations how microbiome interactions via small molecules are shaped by the availability of nutrient resources.